Interview: How can we understand our risk for cancer?
Read Doing Well’s full interview with Dr. Joshua LaBaer, a physician and cancer researcher.
According to the National Cancer Institute, 38.9% of people will be diagnosed with cancer at some point in their lives. This means that cancer will touch all of us: if not personally, then through someone we know or love.
At the same time, the death rate for cancer has decreased since the 1990s. This is largely thanks to advances in cancer treatment and screening, which allows disease to be detected earlier, when it's more treatable. Public education campaigns discouraging smoking (a major cause of lung cancer) and encouraging preventive measures like HPV vaccination (which can prevent cervical and other cancers) have also made a big difference.
I spoke to Dr. Joshua LaBaer—the executive director of the Biodesign Institute at Arizona State University and one of the country’s lead researchers in the field of personalized diagnostics—about why cancer develops and how to understand our risk for the disease. Our conversation has been edited for length and clarity.
Jump ahead:
Mia Armstrong-López: What is cancer?
Joshua LaBaer: Cancer is a series of diseases—there's multiple cancers—in which the cells of our body stop behaving according to the rules for how cells should behave. Ordinarily, in an adult, we get a fixed number of cells. It varies very slightly, but it's a pretty steady state, because cells ordinarily obey the signals that tell them when to divide and when not to divide.
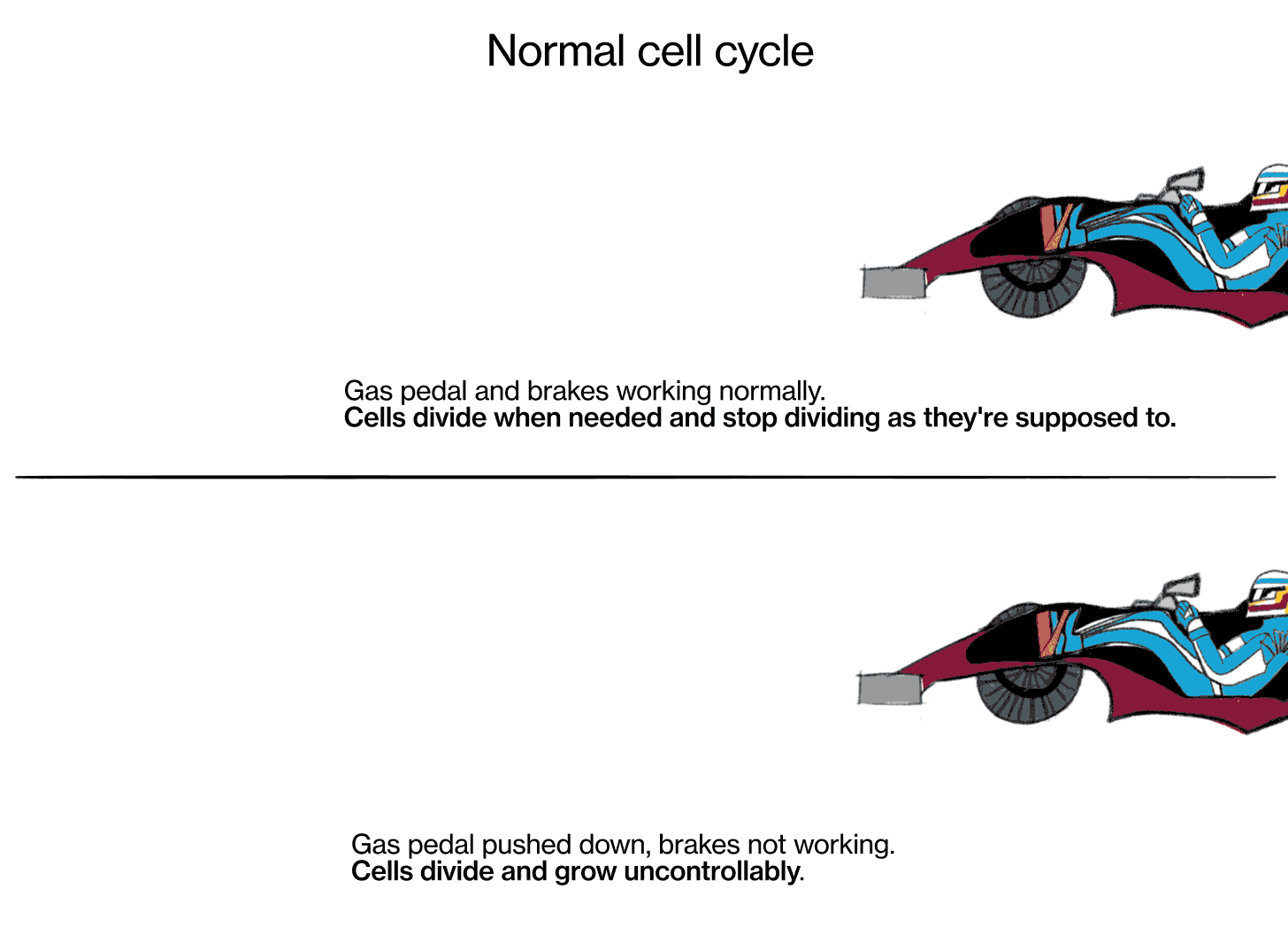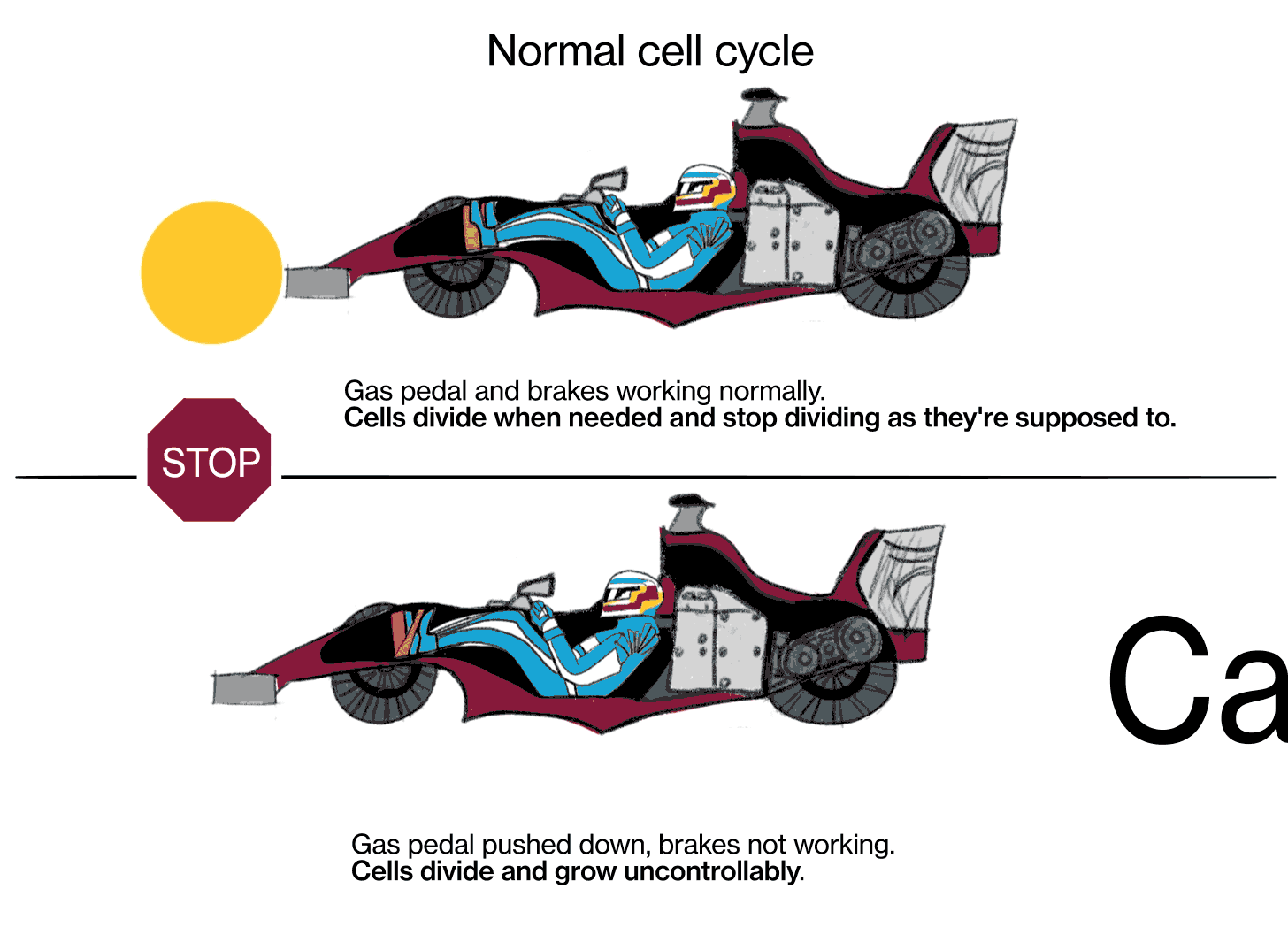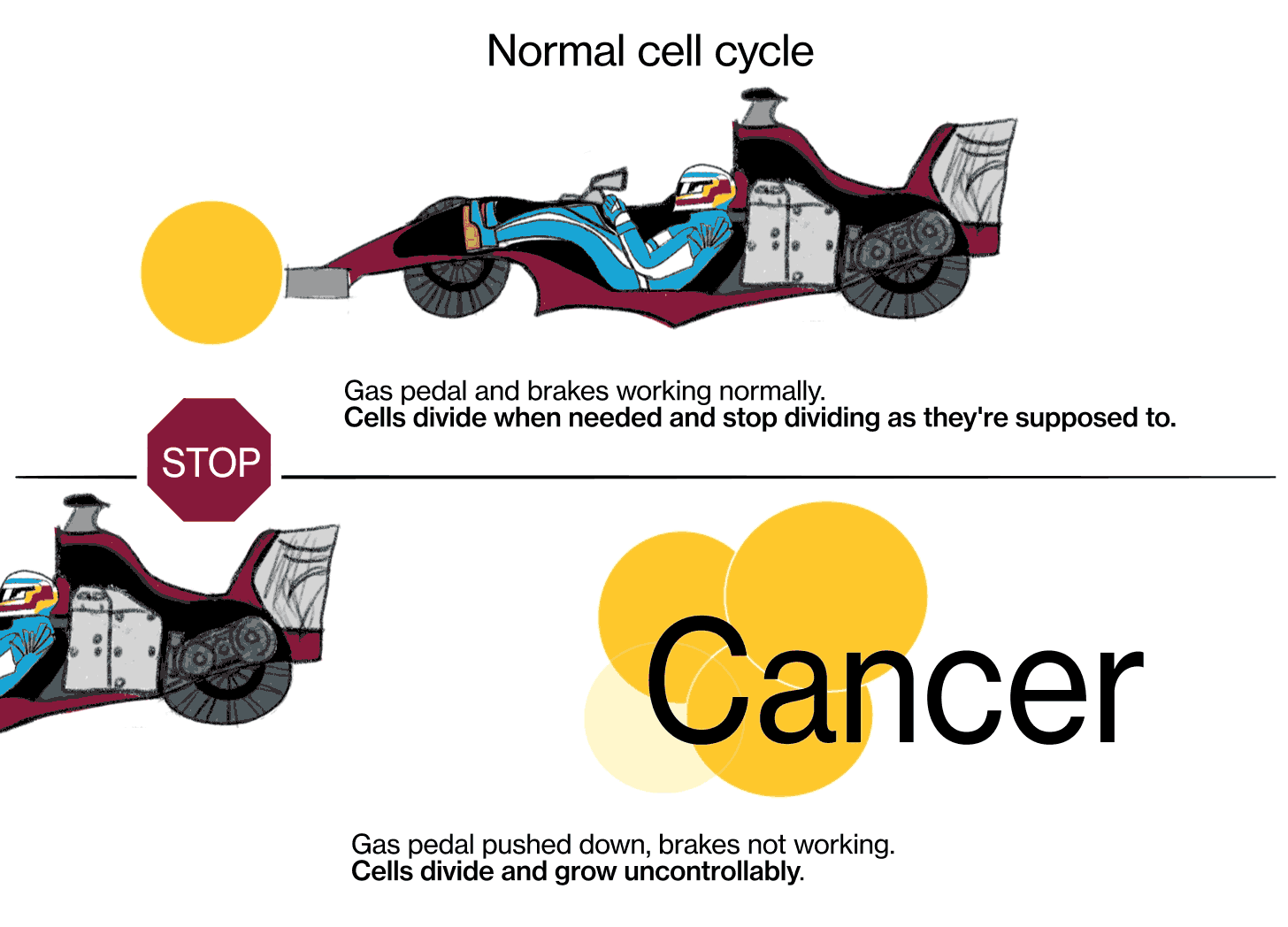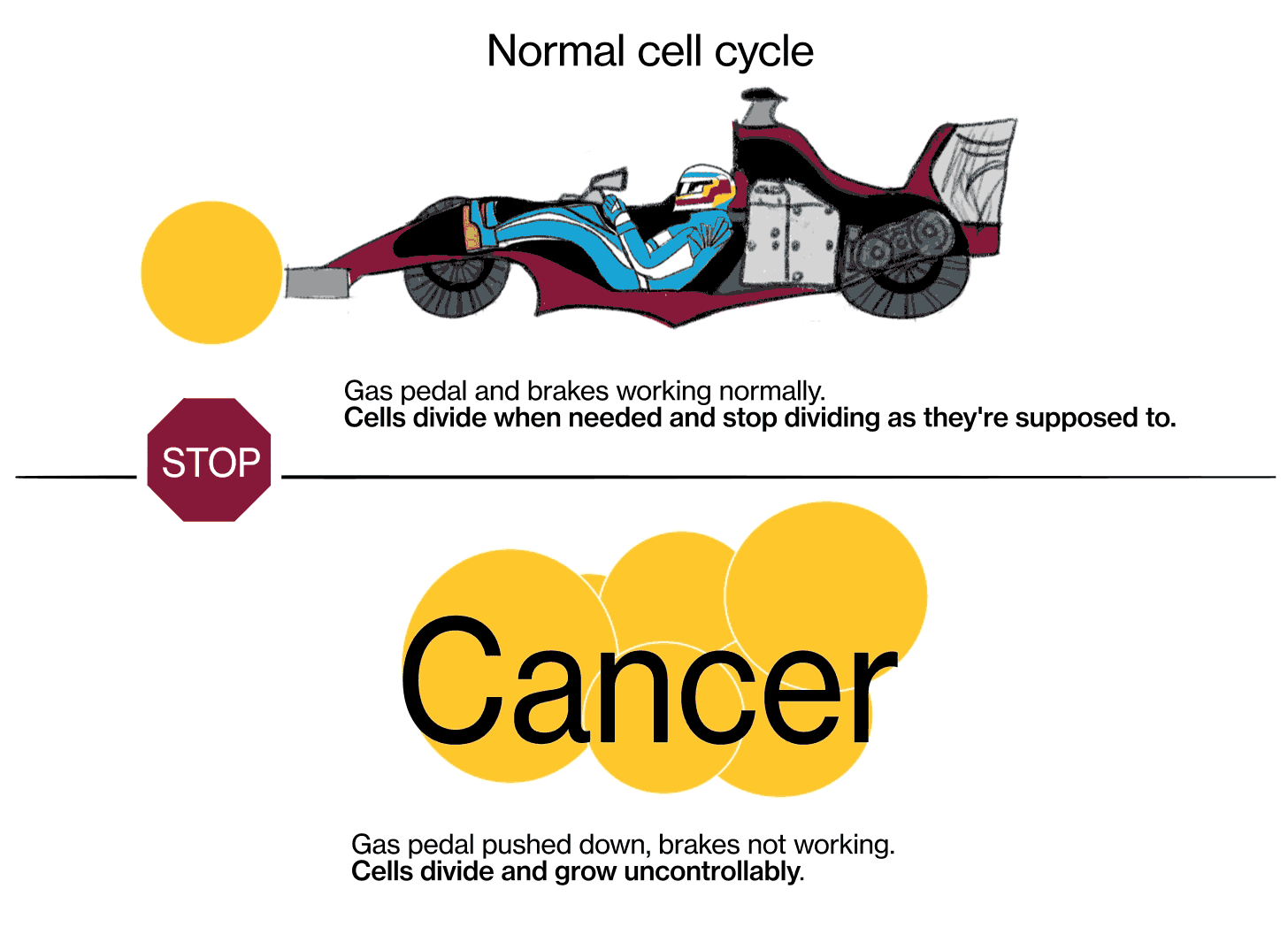
Cancer is a disease where cells don't follow those instructions, and they start to divide out of control. There are really two key characteristics that make cancer cancer. The first is that cells proliferate (multiply) when they should not be proliferating. And the second is that cancer cells don't obey the geographic rules of where they should belong—they start to invade places where they shouldn't be. Sometimes that invasion is local, where the tumor starts, and sometimes they break off, spread throughout the body via the bloodstream or other methods, and grow in other places.
MAL: Why do cells start breaking those rules?
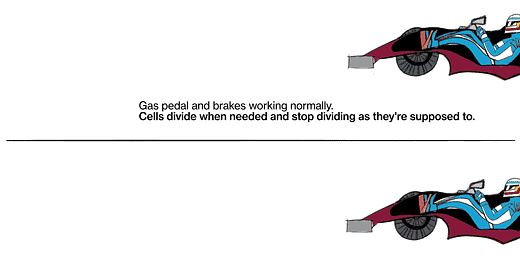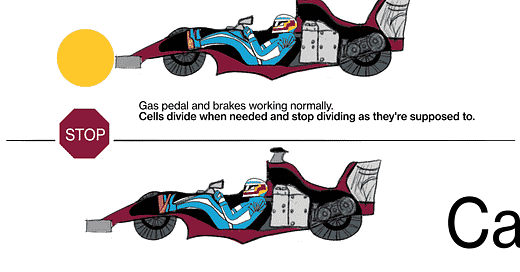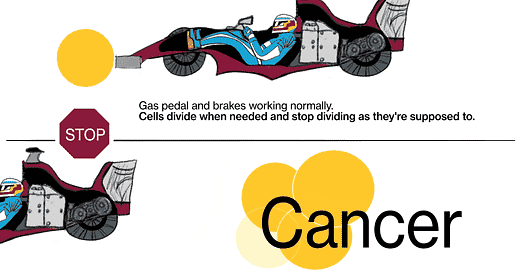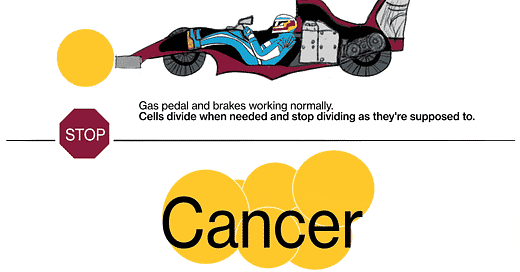
JL: We know that cancer is a genetic disease, that’s to say, genes in these cells get mutated in such a way that the signal to proliferate (multiply) gets turned on and can't be shut off again, kind of like a light switch that's stuck in the “on” position. There are signals in the body that are there to stop cells from proliferating, those are called tumor suppressor genes. [When] those genes fail, their signals are shut off somehow.
The metaphor I often use is: Every cell has elements that look like the accelerator in a car, and elements that look like the brake in a car. The accelerators are like what are called oncogenes, and they get stuck in the on position. The pedal gets pushed to the floor, and it doesn't come back. It's stuck. The brakes are the tumor suppressor genes, and for some reason they fail, and the car starts rolling out of control. That's fundamentally what causes the cells to proliferate when they shouldn't.
MAL: You mentioned that cancer is a genetic disease. What do we know about the genes that cause cancer?
JL: There are two ways that it can happen. One is what's called inherited cancers. There are a number of syndromes in which people are born with mutated genes that predispose them to cancer, often at an early age. A classic example is the BRCA gene—this is a breast cancer gene. Women have a very high chance of getting cancer if they inherit that gene.
Most cancer is not that. Most cancer is what we call sporadic cancer—during the course of a person's lifetime, genes within certain cells will mutate and cause those cells to start to misbehave. We think that probably people are developing cancers often, but most of those cancers get taken care of by our immune systems and other systems in the body that catch them early and basically stop them from becoming full-on cancers. But every once in a while, the right mutations happen in the right genes, and they accumulate. It takes multiple mutations in a cell to lead to cancer; it's not just one gene. Those mutations occur one after the other, not necessarily right away, but over periods of years. And at that point, the cell starts to behave like a cancer cell and starts to grow out of control.
There are probably around 20 or 30 different biochemical pathways that really play a central role in causing cancer. We believe we know most of them at this point. It can be various genes along those pathways that cause the cancer. Some of those pathways are the oncogenes—they're the accelerators that get stuck in the on position. Other pathways include tumor suppressor genes, which are normally the brakes, they tell the cells to stop dividing.
Imagine, for example, if you cut your skin. The cells on the border of that wound are going to start to divide. They're going to sense a signal that says “There's an opening here, I need to close it.” They will grow until they meet up with the cells on the other side. And then these tumor suppressor genes will come in and say, “Okay, it's healed, we don't need any more cell division,” and healthy cells will stop dividing. Tumor cells would keep going, and they just never stop.
There are different genes in different types of cancer. A lung cancer might involve different pathways than a colon cancer or a breast cancer or a brain cancer. You have to study all of those.
MAL: One of the tools we have to detect and monitor cancer are biomarkers. What is a biomarker?
JL: One of the biggest challenges for cancer is that we often detect it too late. Once the cancer escapes the initial tumor and gets out into the body, the chance for long-term survival drops significantly. If a woman is detected with early-stage breast cancer so that the tumor is entirely within her breast, there is a 99% five-year survival. If we catch it early, we can do a lot, but if it's too late, it's much harder to treat.
So the key is, how do we find it early? In the case of breast cancer, there is a tool called mammography, which can look for tumors. It can often determine cancer, but not always. It misses about 25% [of tumors], and it often says that there's cancer when there isn't cancer. In other cancers, we don't even have that, we have no easy way of knowing what's there.
The idea of biomarkers is: If it's possible that cancer is somehow sending out a molecular signal, could we measure something in the blood to say, “Somewhere you might have a cancer, we need to go looking for it”?
A biomarker is any measurement that can lead you to either a medical or biological abnormality. If we can find them in the bloodstream, they may betray the presence of a cancer that we didn't see otherwise. Once we know there's a cancer, we look for molecular signatures that tell us what type of cancer it is, what pathways might be abnormal, and whether or not that cancer would respond or is responding to therapy. Some biomarkers help guide us toward certain medications, they help us determine what's the best therapy.
MAL: If I have a history of cancer in my family, how should I think about my own risk?
JL: You need to look at the characteristics of the history of cancer in your family. For example, if you were to tell me that your mother got breast cancer at age 65, and your uncle got prostate cancer at age 70—you have cancer in your family, but they're getting cancer at the age that people mostly get cancer. And so it would be hard to say that you have a likely inherited higher risk.
Now, if you told me that your mother got cancer when she was 45, and that her sister got cancer when she was 50, and you have a cousin who got cancer when she was 35—when people are getting cancer in your family, and it's the same cancer [for example, breast cancer], and they're getting it at an unusually young age—then I would probably go get tested for BRCA.
The things to be looking for are early age of onset and a common type of cancer. Cancers start to really increase after [age] 50, and particularly in the 55 to 70 range. So in that window, I'd be paying a lot of attention. But the real risk would be if it were a common type of cancer that was occurring particularly in people of young age.
MAL: People hear a lot about things that they should and shouldn't eat, drink, or do to prevent cancer, and it can be easy to get overwhelmed. Help us sort through this: Are there things we can do to lower our risk?
JL: It's complicated, and we don't have any simple answers. I generally tell people that a “heart healthy” diet is not a bad diet for cancer as well. Eating less processed food, trying to start with straightforward natural ingredients, making the food yourself. I would avoid eating too much saturated fat, eating excessive amounts of carbohydrates. Get exercise on a regular basis. You don't have to go run marathons, you just need to get out and move a few times a week. All the general recommendations apply. Personally, I'm a believer in eating a lot of vegetables. When I was in college, I did research on the protective effects of the cruciferous family—broccoli, cauliflower, brussel sprouts, cabbage. Eating some of that is not a bad idea, [but] they're not like magic pills. You want to eat a varied diet of healthy stuff—that's the best I can say.
Mia Armstrong-Lopez: The disease you're describing is so complex. “Eat this one thing” or “take this one pill” to prevent cancer—those simple solutions can't deal with the complexity of the problem.
JL: Nor is that really how cancer starts. Cancer is genes going wrong. It's mutations in genes or or various molecular mechanisms that shut genes on and or off. A lot of it has to do with chance and exposure.
There are certain risky things, without a doubt. It’s almost impossible to overstate how bad smoking is. If you take smoking and you compare it to all of the other risk factors that have been listed to cause cancer or relate to cancer—high-fat diet, plastics, hormones—put those all together, they're not as bad as smoking. So any kind of smoked tobacco product, I would avoid.
MAL: How has research changed cancer treatment?
JL: Back in the day, when I trained as a cancer doctor, the only tools we had were drugs that blocked cell division. We know there's a core machinery that causes cells to divide, and because we didn't know the specific cancer pathways yet, we just shut them down. We used chemotherapies that would target cell division, and because there are normal cells and healthy cells that are supposed to divide at certain times, we were poisoning all of those. So patients would be sick in the hospital, vomiting, losing their hair, their guts would get irritated, their blood immune systems would shut down. We still have to use that a little bit, it hasn't gone away fully—but with these additional targeted therapies that block specific pathways, most don't cause many symptoms at all. We've been able to make cancer care much more tolerable for patients because of these targeted therapies.
MAL: Imagine five years into the future. How do you think the ways that we detect and treat cancer will be different from what we're doing today? Are you hopeful?
JL: I am hopeful. Back in the 1950s when Sidney Farber and others were developing early stage chemotherapies, they would find this drug that would cause a 15% effect, and [another] drug that would cause a 10% effect. And so people would say, “Well, it's not a big enough effect.” But then they said, “What if we put this together with this, together with this, what would happen?” And then suddenly, boom! By adding little bits of things that work and putting them together in the proper ways, you were able to get big effects out of what were initially small effects.
That's been the history of cancer care; it’s just been getting better and better over time. You're seeing mortality drop from cancer since the 1990s, and that's because we're getting better and better at combining things in ways that work. It's not a fast process. It's one that you just kind of work at and work at and work at. And now that we have more tools, and we know more pathways, and we're getting better at the chemistry, I think we're going to get faster. We're going to get better at stopping these cancers.
I'm definitely optimistic. We've done a lot already, and if we can keep studying, we’ll get better and better.
Learn more about ASU’s research on cancer.
Do you have a question or topic you’d like us to tackle? Would you like to share your experience? Reach out at any time—we’d love to hear from you.